This post may refer to COVID-19
To access official information about the coronavirus, access CDC - Centers for Disease Control and Prevention.
www.nature.com
The mechanisms of action of Ivermectin against SARS-CoV-2: An evidence-based clinical review article
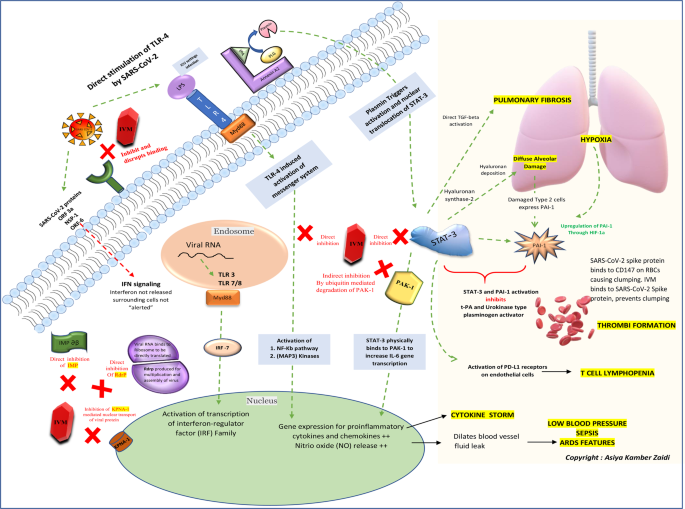
Considering the urgency of the ongoing COVID-19 pandemic, detection of various new mutant strains and future potential re-emergence of novel coronaviruses, repurposing of approved drugs such as Ivermectin could be worthy of attention. This evidence-based review article aims to discuss the mechanism of action of ivermectin against SARS-CoV-2 and summarizing the available literature over the years. A schematic of the key cellular and biomolecular interactions between Ivermectin, host cell, and SARS-CoV-2 in COVID-19 pathogenesis and prevention of complications have been proposed.
Health
Abstract
Considering the urgency of the ongoing COVID-19 pandemic, detection of various new mutant strains and future potential re-emergence of novel coronaviruses, repurposing of approved drugs such as Ivermectin could be worthy of attention. This evidence-based review article aims to discuss the mechanism of action of ivermectin against SARS-CoV-2 and summarizing the available literature over the years. A schematic of the key cellular and biomolecular interactions between Ivermectin, host cell, and SARS-CoV-2 in COVID-19 pathogenesis and prevention of complications have been proposed.
Introduction
A relatively recent surge in zoonotic diseases has been noted over the past few decades. Several reasons could be responsible for this “spill-over” of disease-causing agents from animals to humans. These include an exponential rise in the global population causing man to encroach new ecological habitats in search of space, food, and resources as well as improved opportunities for rampant wildlife trade causing inter-species pathogen jumps. The 1980s was known for HIV/AIDS crisis that originated from the great apes, while the Avian flu pandemic in 2004-07 came from the birds. The pigs lead to the Swine flu pandemic in 2009 and bats were the original hosts of Ebola, Severe Acute Respiratory Syndrome (SARS), Middle Eastern respiratory syndrome (MERS), and probably Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) outbreak as well.
COVID-19 has already caused millions of deaths worldwide and has paralyzed not only the world’s healthcare system but also the political and economic relations between countries [1]. The fact that the SARS-CoV-2 virus has been thought to have originated from wildlife and may have “jumped” into humans, not only highlights future risks from animal-borne diseases but also provides an important clue to its resolution. In such a scenario, where this “jump” has been made from animal to human, it seems only logical to review a drug that has worked efficiently against a disease-causing agent and is available in a form that is safe for human consumption since the early 1980 s.
Ivermectin belongs to a group of avermectins (AVM), which is a group of 16 membered macrocyclic lactone compounds discovered at the Japanese Kitasato institute in 1967 during actinomycetes cultures with the fungus Streptomyces avermitilis [2]. This drug radically lowered the incidence of river blindness and lymphatic filariasis and was discovered and developed by William C. Campbell and Satoshi Ōmura for which they received the Nobel Prize in Physiology or Medicine in 2015 [3, 4]. Ivermectin is enlisted in the World Health Organization’s Model List of Essential Medicines [5].
Drug repurposing, drug redirecting, or drug reprofiling is defined as the identification of novel usages for existing drugs. The development risks, costs as well as safety-related failure, are reduced with this approach since these drugs have a well-established formulation development, in vitro and in vivo screening, as well as pharmacokinetic and pharmacodynamic profiles. Moreover, the first clinical trial phases of many such drugs have been completed and can be bypassed to reduce several years of development. Therefore, drug repurposing has the potential to reduce the time frame for the whole process by up to 3–12 years and carries great potential [6].
Although several drugs received Emergency Use Authorization for COVID-19 treatment with unsatisfactory supportive data, Ivermectin, on the other hand, has been sidelined irrespective of sufficient convincing data supporting its use. Nevertheless, many countries adopted ivermectin as one of the first-line treatment options for COVID-19.
With the ongoing vaccine roll-out programs in full swing across the globe, the longevity of the immunity offered by these vaccines or their role in offering protection against new mutant strains is still a matter of debate. The adoption of Ivermectin as a “safety bridge” by some sections of the population that are still waiting for their turn for vaccination could be considered as a “logical” option.
Several doctor-initiated clinical trial protocols that aimed to evaluate outcomes, such as reduction in mortality figures, shortened length of intensive care unit stay and/or hospital stay and elimination of the virus with ivermectin use have been registered at the US ClinicalTrials.gov [7]. Real-time data is also available with a meta-analysis of 55 studies to date. As per data available on 16 May 2021, 100% of 36 early treatment and prophylaxis studies report positive effects (96% of all 55 studies). Of these, 26 studies show statistically significant improvements in isolation. Random effects meta-analysis with pooled effects using the most serious outcome reported 79% and 85% improvement for early treatment and prophylaxis respectively (RR 0.21 [0.11–0.37] and 0.15 [0.09–0.25]). The results were similar after exclusion based sensitivity analysis: 81% and 87% (RR 0.19 [0.14–0.26] and 0.13 [0.07–0.25]), and after restriction to 29 peer-reviewed studies: 82% and 88% (RR 0.18 [0.11–0.31] and 0.12 [0.05–0.30]). Statistically significant improvements were seen for mortality, ventilation, hospitalization, cases, and viral clearance. 100% of the 17 Randomized Controlled Trials (RCTs) for early treatment and prophylaxis report positive effects, with an estimated improvement of 73% and 83% respectively (RR 0.27 [0.18–0.41] and 0.17 [0.05–0.61]), and 93% of all 28 RCTs. These studies are tabulated in Table 1. The probability that an ineffective treatment generated results as positive for the 55 studies to date is estimated to be 1 in 23 trillion (p = 0.000000000000043). The consistency of positive results across a wide variety of cases has been remarkable. It is extremely unlikely that the observed results could have occurred by chance [8].